Factor Selection: completed fishbone and tips
In the last article, we asked you to determine the potentially significant parameters for a Wittig reaction and provided you with a basic scheme with the main starting materials and product. As you can see from the completed fishbone diagram we identified 18 factors which may influence the outcome of the reaction. The 3 starting materials are listed as well as base type and base equivalents. The need for a base was not explicitly stated on the scheme but from drawing out the mechanism for this reaction it is clear that one is needed. It is always a good idea to look at the mechanism of the reaction you are studying to ensure hidden factors are not missed, or unnecessary factor are not investigated. While the aldehyde and the alkyl halide are fixed to deliver the required product, alternative phosphines could be studied. A mix of chemical and engineering factors are stated as well as some you may not have thought of such as the order, mode and rate of addition of the four reagents.
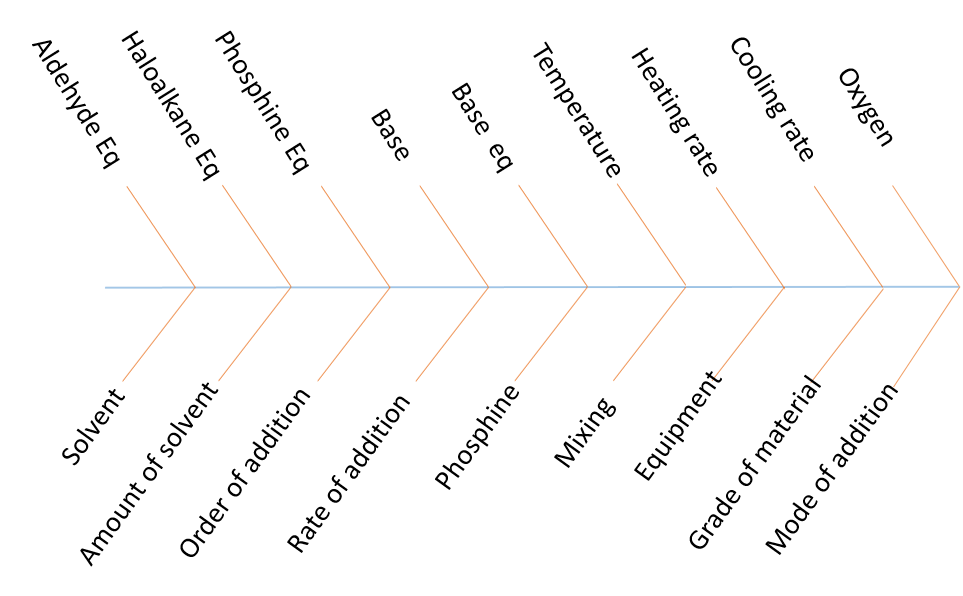
All the steps in the reaction should be considered and all possible factors should initially be highlighted, including the ones you have already discounted as not affecting the reaction. Once you have a complete list of PSPs the choice about which to investigate can be made based on literature knowledge, previous experience or sighting experiments. We have included some points to consider during factor selection below.
- You may find it is easier to work in a group for factor selection, with a mix of chemists and engineers to provide a range of knowledge to the process.
- Factors can be discrete or continuous. A continuous (or quantitative) factor has well defined levels (the range in which you investigate) and can be easily changed e.g. pH, temperature, concentration. All that limits you is what your equipment can achieve. Discrete (or qualitative) factors are non-continuous and limited by their existence, making them slightly more difficult to change between, e.g. solvent, catalyst, ligand or type of equipment.
- Factors which are thought to influence the kinetics of the reaction should be investigated to gain more control over competing pathways within the reaction i.e. directing towards product formation rather than impurity generation. Reaction progress kinetics is used to this end and we generally do not include time as a factor but instead encourage the collection of samples at time points to create reaction profiles. Reaction progress kinetics and reaction profiling to aid process development will be covered in a future post.
- There are scale-dependent and scale-independent factors; potentially significant scale dependent factors (e.g. mass transfer, heat transfer, heating, cooling and addition rates) should be considered initially but looked at in more detail during subsequent experimentation as they are easier to measure and control on a larger scale.
- Consider the reaction and the work up separately. This is also important when setting the response and measuring the output of the reaction. Certain factors which are under investigation in a design will lead to varying work-up methods (e.g. different solvents), meaning the response should be measured before work up occurs.
Hopefully this exercise and the tips above will come in handy the next time you're planning a screening design.